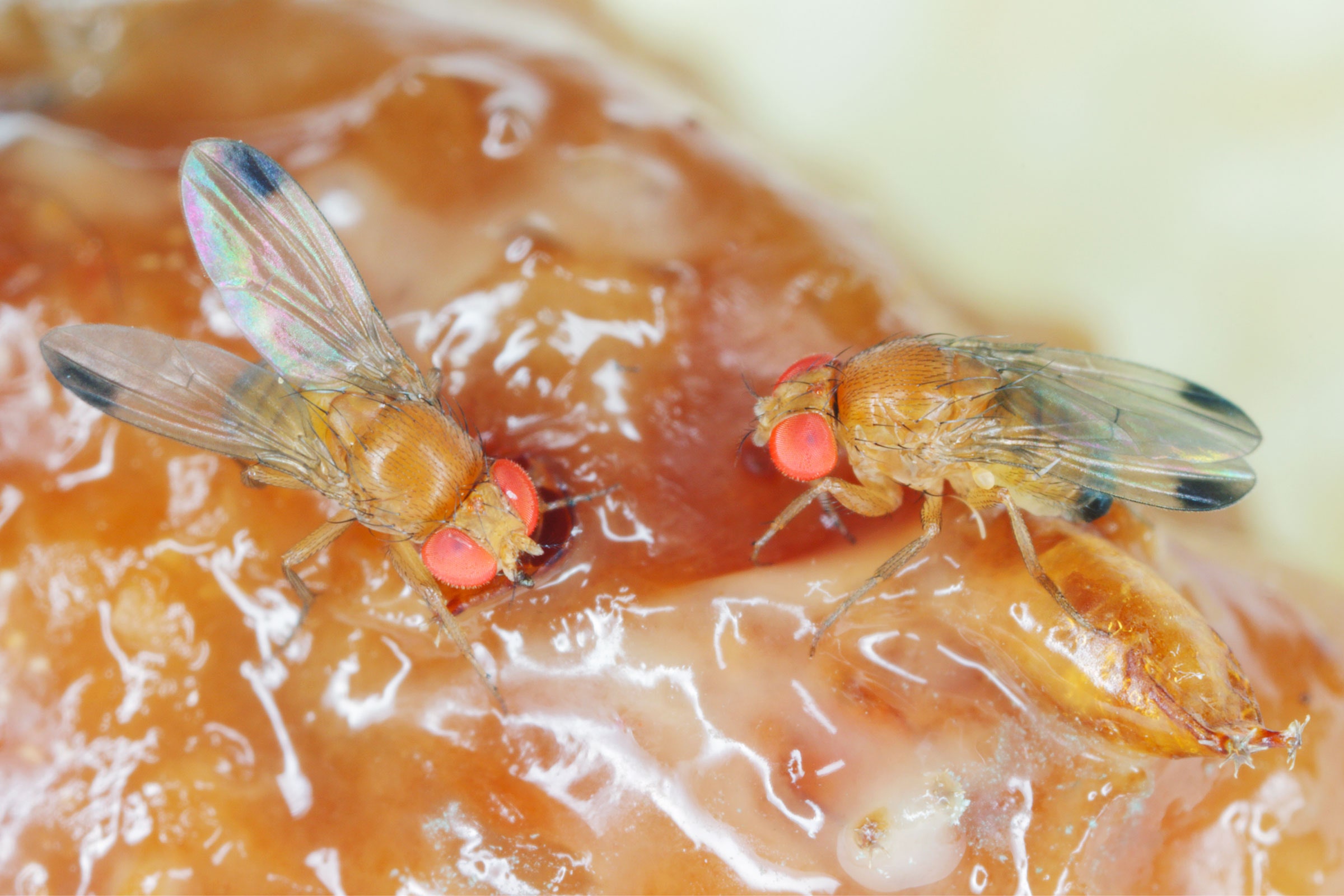
Scientists Are Gene-Editing Flies to Fight Crop Damage
“If you release enough males over a long enough period of time, you can eliminate the pest,” says Omar Akbari, a professor of cell and developmental biology at the University of California, San Diego, who originally developed the gene-editing technique for flies and licensed it to Agragene.
Using sterilization as a way to control insect pests isn’t a new idea. Since the 1950s, the US government has used it to tamp down screwworms, parasitic flies that feed on the flesh of livestock. Sterilized male screwworms are released to mate with females, which lay eggs that don’t hatch. This technique has led to their eradication in North and Central America. But those male insects are made sterile with radiation rather than genetic engineering. The downside is that high levels of radiation impair their ability to reproduce, meaning dozens of insects are often released for every wild one.
Meanwhile, researchers at North Carolina State University are developing an alternative control method that wouldn’t require repeated releases. In the lab, they used a technique called a gene drive to make female offspring unable to reproduce. Gene drives are designed to preferentially spread or “drive” certain genetic traits throughout a population, overruling the rules of heredity.
In a paper published earlier this month, entomologist Max Scott and his team described using Crispr to inactivate a region of the doublesex gene, which is required for female sexual development. The researchers injected Crispr into fly embryos, along with a fluorescent protein so they could track which flies ended up with the desired change.
When the flies matured, the researchers mated the engineered insects with wild ones that didn’t have the mutated doublesex gene. Normally, about 50 percent of offspring would be expected to inherit the change. But with the gene drive, 94 to 99 percent of the flies’ offspring ended up with it. Females that inherited the mutated gene were sterile and unable to lay eggs. But male offspring, which also carry the change, remain fertile. Unlike Agragene’s approach, the males go on to breed and pass on the trait to subsequent generations. This allows the gene to continue spreading—and knocking out future generations of flies—without the need to release more insects.
“If we're trying to do population control, you really want to target a gene that's required for female reproduction, because it's the female that is producing the next generation,” Scott says.
The researchers used mathematical modeling to predict how well the gene drive system would suppress populations of flies kept in laboratory cages, and found that releasing one modified fly for every four wild ones could tank populations within eight to 10 generations. (Each generation lasts about two weeks, so crashing a lab population could take around 20 weeks.) Now the team is conducting experiments using cages of real gene-edited flies to learn whether the gene drive will suppress their numbers as the modeling predicts.
Researchers elsewhere are developing gene drives as a way to control disease-carrying mosquitoes, but none of these insects have been released yet in the wild. “There are a lot of risks associated with it, because it’s a technology that you can’t control once you release it,” says Akbari, whose lab has also worked on gene drives for spotted-wing drosophila. One risk is the possibility of spillover: If the target species mates with a closely related one, it could transfer the gene drive to a native or valued species.